Acidum Nucleicum Mycobacterii Tuberculosis et Rifampicinum (RIF), Resistentia (INH)
Nomen producti
HWTS-RT147 Mycobacterium Tuberculosis Acidum Nucleicum et Rifampicinum (RIF), (INH) Detectionis Instrumentum (Curva Liquefactionis)
Epidemiologia
Mycobacterium tuberculosis, breviter Tubercle bacillus (TB) appellatum, est bacterium pathogenicum quod tuberculosin efficit, et nunc medicamenta antituberculosa primae lineae vulgo adhibita sunt isoniazidum, rifampicinum et ethambutolum, cetera.[1]Attamen, propter usum improprium medicamentorum antituberculosi et proprietates structurae parietis cellularis ipsius Mycobacterium tuberculosis, Mycobacterium tuberculosis resistentiam medicamentis antituberculosis evolvit, et forma praesertim periculosa est tuberculosis multiresistens (MDR-TB), quae resistit duobus medicamentis communissimis et efficacissimis, rifampicino et isoniazido.[2].
Problema resistentiae medicamentorum contra tuberculoseum in omnibus terris a OMS inquisitis exstat. Ut accuratiores curationes aegrotis tuberculosis praebeantur, necesse est resistentiam medicamentis antituberculosis, praesertim resistentiam rifampicini, deprehendere, quae gradus diagnosticus a OMS in curatione tuberculosis commendatus factus est.[3]Quamquam detectio resistentiae rifampicini fere aequivalet inventioni multiresistentis tuberculosis (MDR-TB), sola detectio resistentiae rifampicini ignorat aegros cum INH monoresistente (referente resistentiam ad isoniazidum sed sensibili ad rifampicinum) et rifampicino monoresistente (sensibilitate ad isoniazidum sed resistentia ad rifampicinum), quod ad aegros subiciendas initialibus curationibus iniustis ducere potest. Ergo, probationes resistentiae isoniazidi et rifampicini sunt requisita minima necessaria in omnibus programmatibus moderationis DR-TB.[4].
Parametri Technici
| Repositorium | ≤-18℃ |
| Tempus conservationis | Duodecim menses |
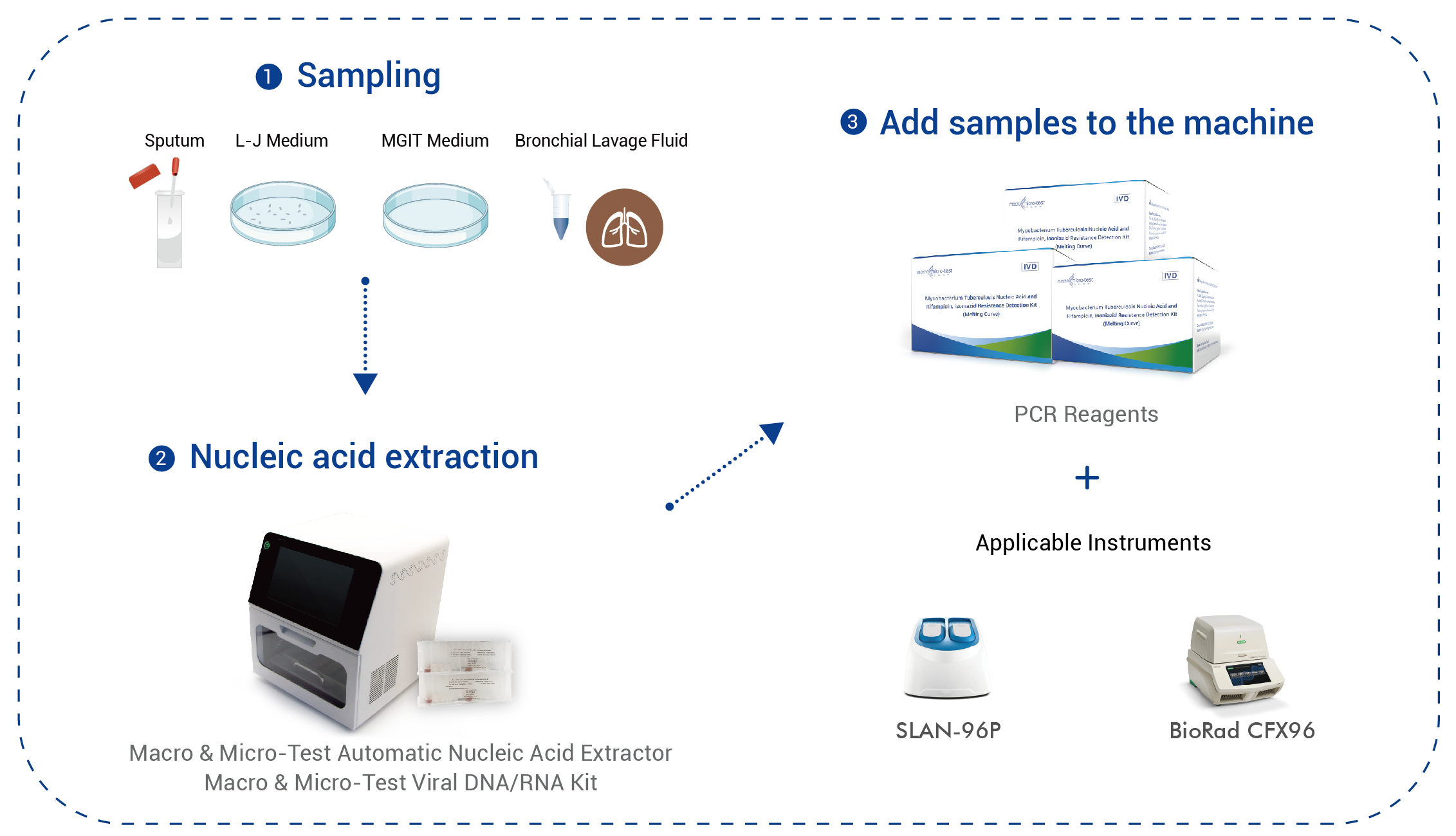
| Typus Speciminis | Exemplum sputi, cultura solida (medium LJ), cultura liquida (medium MGIT) |
| CV | <5.0% |
| Limitatio Detectionis (vel Limitatio Detectionis) | Limita de detectionis (LoD) instrumenti ad Mycobacterium tuberculosis detegendum est 10⁵ bacteria/mL;Limita de detectionis (LoD) instrumenti ad rifampicinum typum naturalem et typum mutantem detegendum est 150 bacteria/mL; Limita de detectionis (LoD) instrumenti ad isoniazidum typum naturalem et typum mutantem detegendum est 200 bacteria/mL. |
| Specificitas | 1) Nulla reactio transversalis fit cum apparatu adhibita est ad detegendum DNA genomicum humanum (500ng), alia 28 genera pathogenorum respiratoriorum, et 29 genera mycobacteriorum non tuberculosorum (ut in Tabula 3 demonstratur).2) Nulla reactio transversa fit cum apparatu adhibita est ad detegendos locos mutationis aliorum genorum resistentium medicamentis Mycobacterium tuberculosis sensibilium rifampicino et isoniazido (ut in Tabula 4 demonstratur).3) Substantiae communes interferentes in exemplaribus examinandis, ut rifampicinum (9mg/L), isoniazidum (12mg/L), ethambutolum (8mg/L), amoxicillinum (11mg/L), oxymetazolinum (1mg/L), mupirocinum (20mg/L), pyrazinamidum (45mg/L), zanamivir (0.5mg/L), dexamethasonum (20mg/L), nullum effectum in eventus probationis instrumenti habent. |
| Instrumenta Applicabilia | SLAN-96P Systema PCR Temporis Realis (Societas Technologiae Medicae Hongshi, Ltd.), Systema PCR Temporis Realis BioRad CFX96 |
Solutio PCR Totalis