Tuberculosis (TB), per Mycobacterium tuberculosis causata, manet periculum salutis globalis.Et crescens resistentia clavibus TB medicamentis sicut Rifampicin (RIF) et Isoniazid(INH) est critica et ortu impedimentum globalis TB nisus dicionis.Celeri et accuratae hypotheticae experimenti TB et resistentiae RIF& INH commendatur ab QUI ut aegros infectos mature cognoscant et opportunis in tempore curationi praebeant.
Provocationes
Aestimatum 10.6 miliones hominum cum TB 2021 cum augmento 4.5% decies centena millia in 2020 inciderunt, inde in extimationis 1.3 miliones mortuorum, pares 133 casibus per 100,000.
Medicamento repugnans TB, praecipue MDR-TB (repugnans RIF & INH), magis magisque afficit tractationem globalem TB ac praeventioni.
Celeri simultanea TB et RIF/INH resistentia diagnosis instanter requisita est ad tractationem maturiorem et efficaciorem comparatam cum medicamento morante susceptibilitatem probationis proventuum.
Our Solution
Marco & Micro-test's 3-in-1 TB Deprehensio pro TB contagio / RIF & NIH Resistentia Detectio Kitefficit diagnosis efficientis TB et RIF/INH in una detectione.
Curvae technologiae liquescens simul detectionem TB et MDR-TB cognoscit.
3-in-1 TB/MDR-TB deprehensio TB infectio et clavis primae lineae medicamento determinans (RIF/INH) resistentia opportune ac accuratam TB tractationem praebet.
Mycobacterium Tuberculosis Nucleic Acidum et Rifampicin, Isoniazid Resistentia Detection Kit (Curve liquefaciens)
Feliciter cognoscit triplicem TB probationem (infectio TB, RIF & NIH Resistentia) in una detectione!
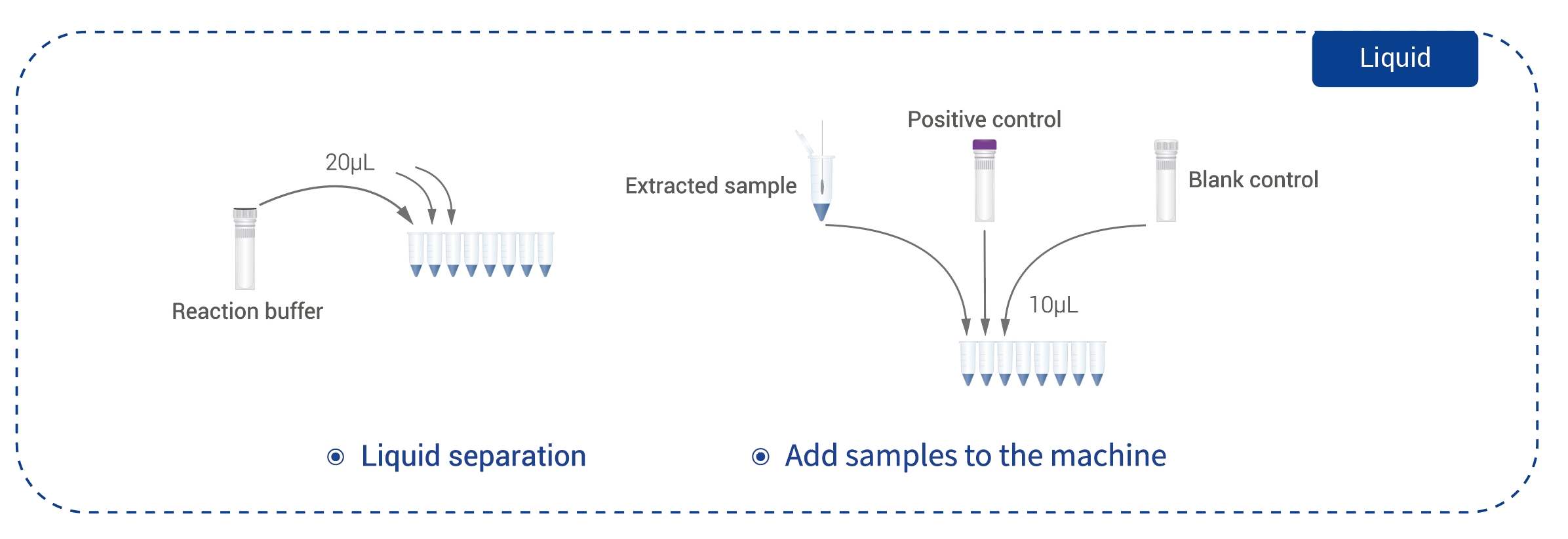
Celerieventum:Available in 1.5-2 hrs latis resultat interpretatio technicae artis operandi extenuando;
Test Sample:1-3 mL sputum;
Alta Sensibilitas:Sensus analyticus 50 bacteria/mL pro TB et 2x103bacteria/mL pro RIF/INH bacteria resistentia, certas deprehensiones etiam in oneribus bacterialibus obtinent.
Multiplex Targets: TB-IS6110;RIF -rpoB-resistentia (507~503);
INH-resistance- InhA/AhpC/katG 315;
Qualitas Validation:Cellula control pro qualitate sanationis specimen ad negationes falsas reducendas;
Wide Compatibility: Compatibility with most important PCR systems for late lab accessibility;
WHO Guidelines Obsequium: adhaerens QUI guidelines pro administratione tuberculosis pharmacopolae reluctans, prospiciendo fidem et congruentiam in praxi clinica.
Opus O
Post tempus: Feb-01-2024
