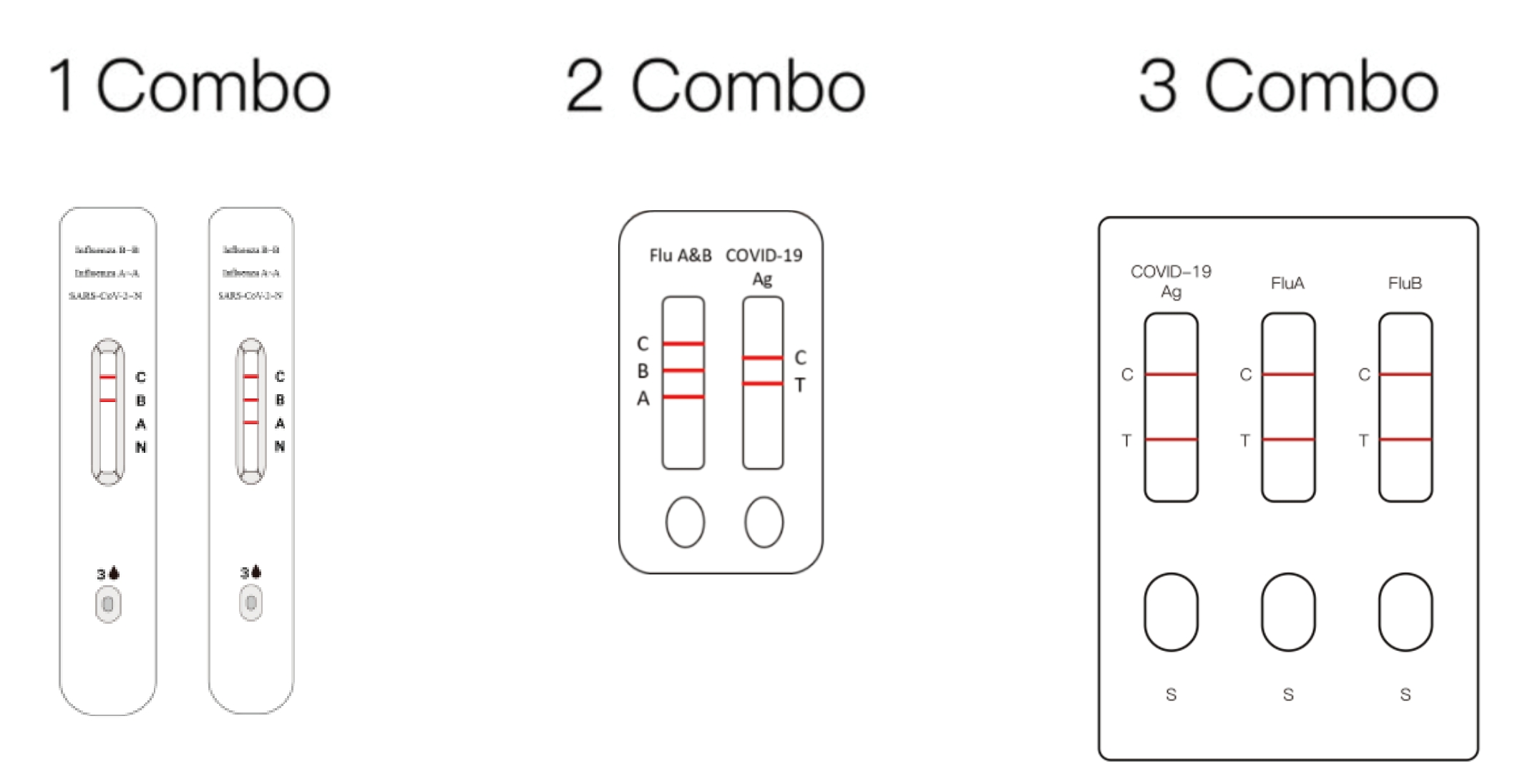
Instrumentum Compositum COVID-19, Influenzae A et Influenzae B
Nomen producti
Instrumentum Detectionis Antigeni Influenzae A/B HWTS-RT098-SARS-COV-2 et Influenzae A/B (Immunochromatographia)
HWTS-RT101-SARS-COV-2, Instrumentum Detectionis Antigeni Influenzae A et B Coniunctum (Immunochromatographia)
Certificatum
CE
Epidemiologia
Morbus Coronavirus 2019 (COVID-19), est pneumonia causata ab infectione novo virus.Coronavirus, nomine "Severe Acute Respiratory Syndrome Corona-Virus 2" (SARS-CoV-2), appellatur. SARS-CoV-2 est novum coronavirus generis β, particulas involutas rotundas vel ovales habens, diametro ab 60 nm ad 140 nm. Homo plerumque SARS-CoV-2 obnoxius est. Fontes infectionis principales sunt aegroti COVID-19 confirmati et vectores asymptomatici SARSCoV-2.
Influenza ad familiam orthomyxoviridarum pertinet et est virus RNA segmentatum negativum filum. Secundum differentiam antigenicitatis inter proteinum nucleocapsidae (NP) et proteinum matricis (M), virus influenzae in tres typos dividuntur: A, B et C. Virus influenzae his annis reperti in typum D classificabuntur. Influenza A et influenza B sunt pathogena principalia influenzae humanae, quae notas latae praevalentiae et infectivitatis validae habent. Infectionem gravem in pueris, senibus et hominibus cum functione immunitatis debilitata causare possunt.
Parametri Technici
| Temperatura repositionis | 4 - 30℃ in statu sicco et obsignato |
| Genus exempli | Peniculus nasopharyngeus, peniculus oropharyngeus, peniculus nasalis |
| Tempus conservationis | XXIV menses |
| Instrumenta auxiliaria | Non requiritur |
| Consumbilia Addita | Non requiritur |
| Tempus detectionis | XV-XX minuta |
| Specificitas | Nulla reactio transversalis cum pathogenis ut coronavirus humanum HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, virus syncytiale respiratorium typi A, B, virus parainfluenzae typi 1, 2, 3, rhinovirus A, B, C, adenovirus 1, 2, 3, 4, 5, 7, 55, Chlamydia pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Neisseria meningitidis, Staphylococcus aureus, Streptococcus pneumoniae et aliis pathogenis existit. |
Fluxus Operis
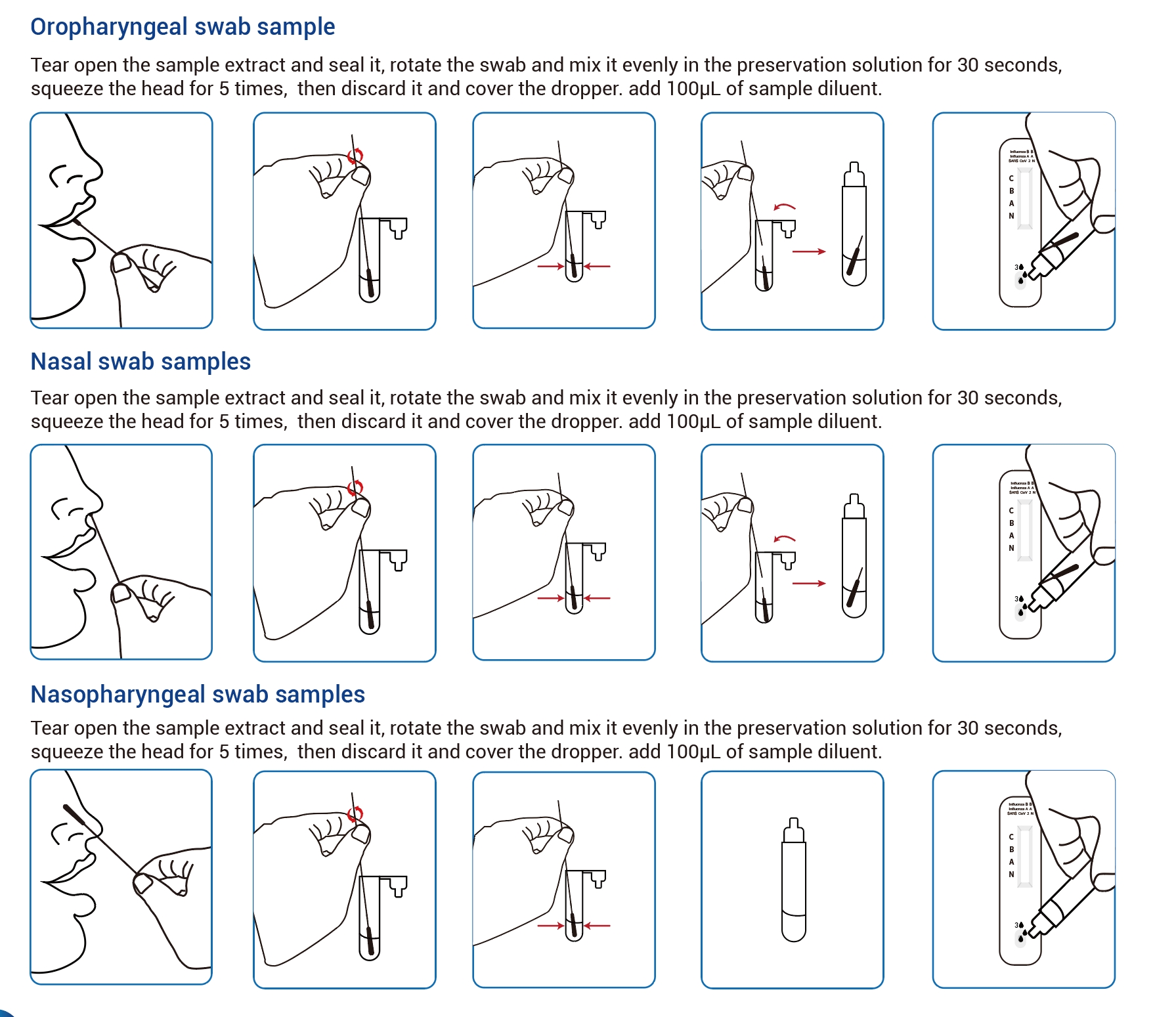
Partes principales