Pathogena Respiratoria Coniuncta
Pathogena Respiratoria Coniuncta Detalia:
Nomen producti
HWTS-RT050-Sex Genera Pathogenorum Respiratoriorum Acidi Nucleici Detegendi Instrumentum(PCR fluorescens)
Epidemiologia
Influenza, vulgo "influenza" appellata, est morbus infectiosus respiratorius acutus a viro influenzae causatus, quod valde contagiosum est et praecipue per tussim et sternutationem transmittitur.
Virus syncytialis respiratorius (VRS) est virus RNA, ad familiam Paramyxoviridarum pertinens.
Adenovirus humanum (HAdV) est virus DNA duplici catena sine involucro. Saltem 90 genotypi inventa sunt, qui in 7 subgenera AG dividi possunt.
Rhinovirus humanum (HRV) est membrum familiae Picornaviridae et generis Enterovirus.
Mycoplasma pneumoniae (MP) est microorganismus pathogenicus cuius magnitudo inter bacteria et virus est.
Canalis
| Canalis | PCR-Mix A | PCR-Mix B |
| Canalis FAM | IFV A | HAdV |
| Canalis VIC/HEX | HRV | IFV B |
| Canalis CY5 | RSV | MP |
| Canalis ROX | Imperium Internum | Imperium Internum |
Parametri Technici
| Depositorium | -18℃ |
| Tempus conservationis | Duodecim menses |
| Typus Speciminis | Peniculus oropharyngeus |
| Ct | ≤35 |
| Limitatio Detectionis (vel Limitatio Detectionis) | 500 Exemplaria/mL |
| Specificitas | 1.Resultata probationis reactionis transversalis demonstraverunt nullam reactionem transversalem inter apparatum et coronavirus humanum SARSr-CoV, MERSr-CoV, HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, virus Parainfluenzae typi 1, 2, et 3, Chlamydia pneumoniae, metapneumovirus humanum, Enterovirus A, B, C, D, virus Epstein-Barr, virus morbillorum, cytomegalovirus humanum, Rotavirus, Norovirus, virus parotitidis, virus Varicellae-zoster, Legionella, Bordetella pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Candida albicans, Candida glabrata, Pneumocystis jiroveci, Cryptococcus neoformans et genomica humana exstitisse. acida nucleica. 2.Facultas anti-interferentiae: Mucinum (60mg/mL), 10% (v/v) sanguinis humani, phenylephrinum (2mg/mL), oxymetazolinum (2mg/mL), natrii chloridum (cum conservativis) (20mg/mL), beclomethasonum (20mg/mL), dexamethasonum (20mg/mL), flunisolidum (20μg/mL), triamcinoloni acetonidum (2mg/mL), budesonidum (2mg/mL), mometasonum (2mg/mL), Fluticasonum (2mg/mL), histamini hydrochloridum (5mg/mL), alpha-interferonum (800IU/mL), zanamivirum (20mg/mL), ribavirinum (10mg/mL), oseltamivirum (60ng/mL), peramivirum (1mg/mL), lopinavirum (500mg/mL), ritonavirum. Substantiae interferentes (60mg/mL), mupirocinum (20mg/mL), azithromycinum (1mg/mL), cefprozilum (40μg/mL), Meropenemum (200mg/mL), levofloxacinum (10μg/mL), et tobramycinum (0.6mg/mL) ad experimentum interferentiae selectae sunt, et eventus demonstraverunt substantias interferentes, supra dictis concentrationibus, nullam reactionem interferentiae ad eventus experimentorum pathogenorum habuisse. |
| Instrumenta Applicabilia | Systema PCR Temporis Realis 7500 Biosystems Applied Systema PCR Temporis Realis Celeris Applied Biosystems 7500 QuantStudio®Quinque Systemata PCR Temporis Realis Systema PCR Temporis Realis SLAN-96P Cyclus Lucis®Systema PCR in Tempore Reali 480 Systema Detectionis PCR in Tempore Reali LineGene 9600 Plus MA-6000 Cyclator Thermalis Quantitativus Temporis Realis Systema PCR Temporis Realis BioRad CFX96, Systema PCR Temporis Realis BioRad CFX Opus 96 |
Solutio PCR Totalis
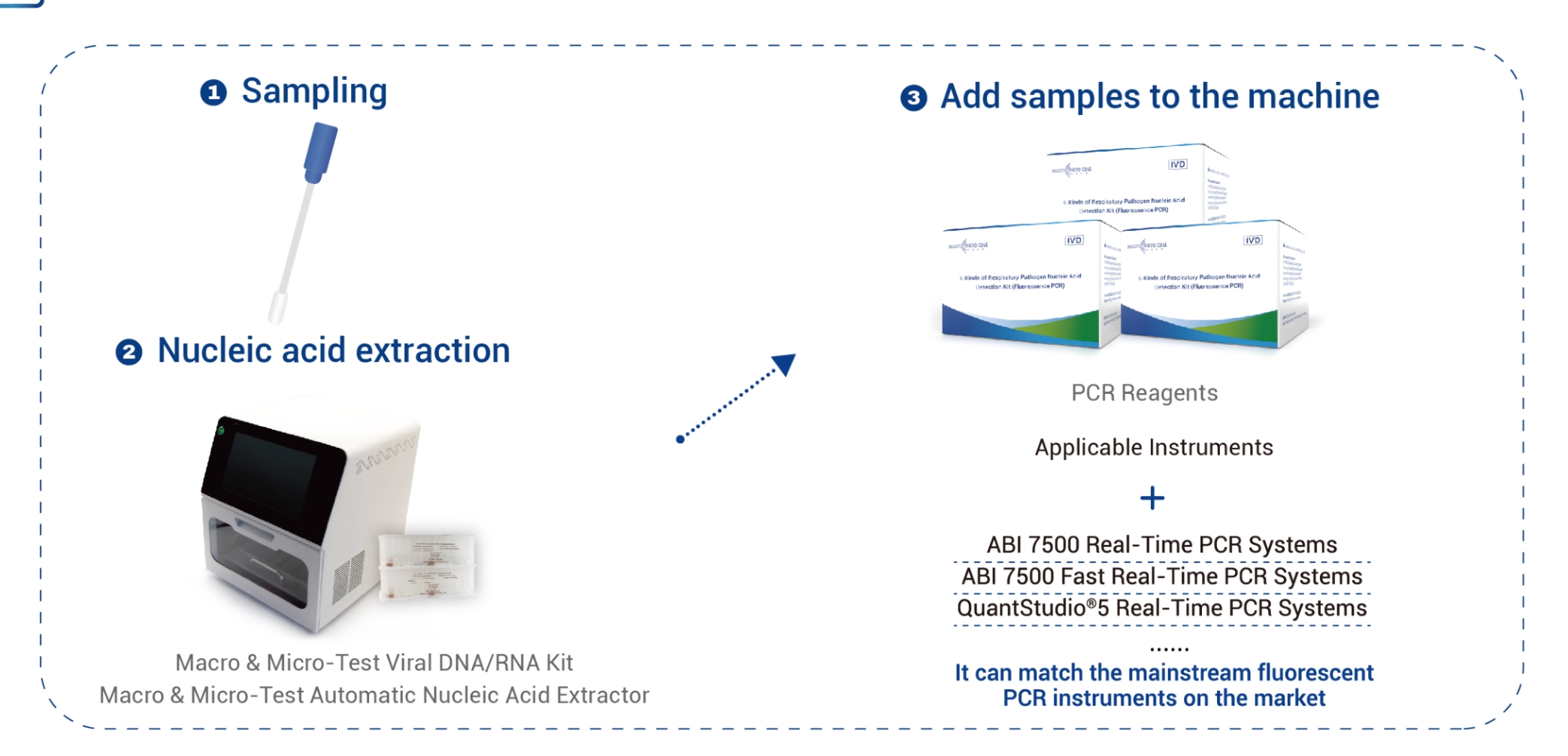
Imagines singularum rerum:

Index Productuum Pertinens:
Quod ad pretia acriora attinet, credimus vos longe lateque quaesituros esse quicquam quod nos superare possit. Certissime affirmare possumus, propter tantam qualitatem et tales pretia, nos infimos fuisse in re Pathogenorum Respiratoriorum Coniunctorum. Hoc productum toto orbe terrarum, ut Hispalis, Europae, Mauritiis, suppeditabitur. Si, postquam indicem productorum inspexeris, de unoquoque producto nostro cupis, libenter nobiscum contactum fac si quaeris. Epistulas electronicas nobis mittere et nobiscum ad consultationem communicare potes, et quam primum tibi respondebimus. Si tibi commodum est, inscriptionem nostram in pagina nostra interretiali invenire et ad officinam nostram venire potes, vel plura de productis nostris ipse scire. Semper parati sumus diuturnas et stabiles necessitudines cooperationis cum quibusvis clientibus potentialibus in campis conexis construere.
In genere, omnibus aspectibus, vili pretio, qualitate, celeritate traditionis et bono stilo producti contenti sumus, cooperationem subsequentem habebimus!