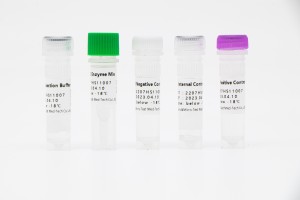
Acidum Nucleicum SARS-CoV-2
Nomen producti
HWTS-RT095 - Instrumentum Detectionis Acidi Nucleici fundatum in Amplificatione Isothermica Specillorum Enzymaticorum (EPIA) pro SARS-CoV-2
Certificatum
CE
Canalis
| FAM | Genum ORF1ab et genum N SARS-CoV-2 |
| ROX | Imperium Internum |
Parametri Technici
| Depositorium | Liquidum: ≤-18℃ in tenebris; Lyophilizatum: ≤30℃ in tenebris |
| Tempus conservationis | Novem menses |
| Typus Speciminis | Specimina ex penicillo pharyngeo |
| CV | ≤10.0% |
| Tt | ≤40 |
| Limitatio Detectionis (vel Limitatio Detectionis) | 500 Exemplaria/mL |
| Specificitas | Nulla reactio transversa cum pathogenis ut coronavirus humanum SARSr-CoV, MERSr-CoV, HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63, H1N1, novum virus influenzae typi A H1N1 (2009), virus influenzae seasonalis H1N1, H3N2, H5N1, H7N9, Influenza B Yamagata, Victoria, virus syncytiale respiratorium A, B, virus parainfluenzae 1, 2, 3, rhinovirus A, B, C, adenovirus 1, 2, 3, 4, 5, 7, typus 55, metapneumovirus humanum, enterovirus A, B, C, D, metapneumovirus humanum, virus Epstein-Barr, virus morbillorum, cytomegalovirus humanum, rotavirus, norovirus, virus parotitidis, virus herpes varicella-banded, Mycoplasma pneumoniae, ... Chlamydia pneumoniae, Legionella, Bacillus pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Bacterium Candidae albicans, Candida glabrata et Cryptococcus neoformans. |
| Instrumenta Applicabilia: | PCR Temporis Realis Applicata Biosystems 7500 SystemaSystema PCR Temporis Realis SLAN ® -96P Systema Detectionis Isothermicae Fluorescentis in Tempore Reali Facile Amp (HWTS1600) |
Fluxus Operis
Optio 1.
Reagens extractionis commendatum: Instrumentum Macro & Micro-Test Viral DNA/RNA (HWTS-3001, HWTS-3004-32, HWTS-3004-48) et Extractor Automaticus Acidi Nucleici Macro & Micro-Test (HWTS-3006).
Optio II.
Reagens extractionis commendatum: Reagens Extractionis vel Purificationis Acidi Nucleici (YDP302) a Tiangen Biotech (Beijing) Co., Ltd.