Antigenum Virus SARS-CoV-2 – Examen Domesticum
Nomen producti
HWTS-RT062IA/B/C-SARS-CoV-2 Virus Antigeni Detection Kit (colloidal gold method) -Nasal
Certificatum
CE1434
Epidemiologia
Morbus Coronavirus 2019 (COVID-19) est pneumonia causata ab infectione novi coronaviri, nomine Syndroma Respiratorium Acutu Gravi Corona-Virus 2 (SARS-CoV-2). SARS-CoV-2 est novum coronavirum generis β, particulas involutas rotundas vel ovales, diametro ab 60 nm ad 140 nm habens. Homo plerumque obnoxius est SARS-CoV-2. Fontes infectionis principales sunt aegroti COVID-19 confirmati et vectores asymptomatici SARSCoV-2.
Studium clinicum
Efficacia Instrumenti Detectionis Antigeni in 554 aegris ex penicillis nasalibus a suspectis symptomaticis COVID-19 intra 7 dies post initium symptomatum collectis, comparata cum examine RT-PCR, aestimata est. Efficacia Instrumenti Probationis Antigeni SARS-CoV-2 haec est:
| Antigenum Virus SARS-CoV-2 (reagens investigationis) | Reagens RT-PCR | Summa | |
| Positivum | Negativum | ||
| Positivum | 97 | 0 | 97 |
| Negativum | 7 | 450 | 457 |
| Summa | 104 | 450 | 554 |
| Sensibilitatem | 93.27% | CI 95.0% | 86.62% - 97.25% |
| Specificitas | 100.00% | CI 95.0% | 99.18% - 100.00% |
| Summa | 98.74% | CI 95.0% | 97.41% - 99.49% |
Parametri Technici
| Temperatura repositionis | 4℃-30℃ |
| Genus exempli | Exempla ex penicillo nasali |
| Tempus conservationis | XXIV menses |
| Instrumenta auxiliaria | Non requiritur |
| Consumbilia Addita | Non requiritur |
| Tempus detectionis | XV-XX minuta |
| Specificitas | Nulla reactio transversa cum pathogenis ut Coronavirus humanum (HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63), Nova influenza A H1N1 (2009), Influenza seasonalis A (H1N1, H3N2, H5N1, H7N9), Influenza B (Yamagata, Victoria), Virus syncytialis respiratorius A/B, Virus parainfluenzae (1, 2 et 3), Rhinovirus (A, B, C), Adenovirus (1, 2, 3, 4, 5, 7, 55) existit. |
Fluxus Operis
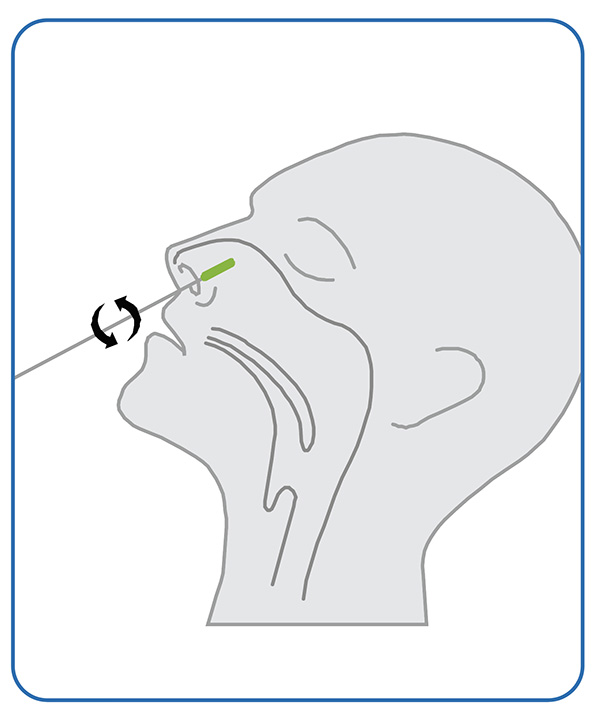
1. Exemplum
●Leniter totum apicem mollem penicilli (plerumque dimidiam ad tres quartas partes unciae) in unam narem inser. Media pressione utens, penicillum contra omnes parietes interiores naris frica. Saltem quinque circulos magnos fac. Et utraque naris per quindecim circiter secundas detergenda est. Eodem penicillo utens, idem in altera nare repete.
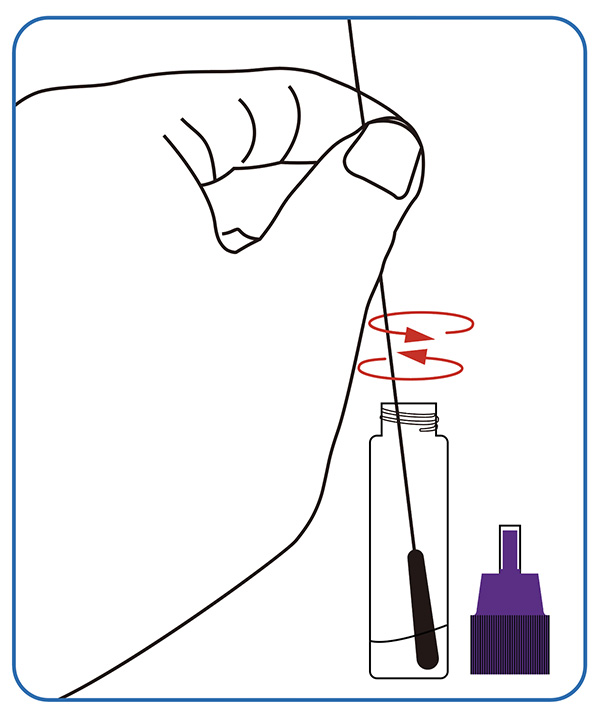
●Dissolutio speciminis.Peniculum penitus in solutionem extractionis speciminis immerge; bacillum peniculi ad punctum fracturae frange, fine molli in tubo relicto. Operculum adstringe, decies inverte et tubum in loco stabili pone.
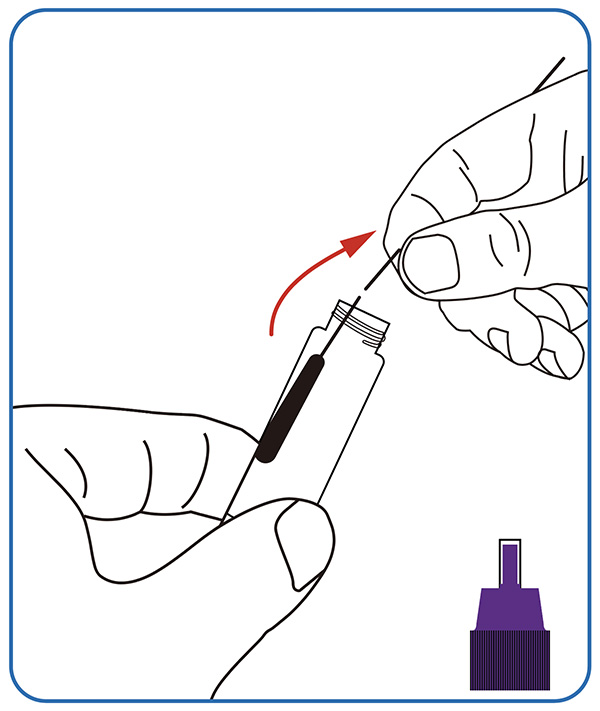
2. Experimentum perage.
Tres guttas exempli processati extracti in foramen exempli chartae detectionis infunde, operculum contorque.
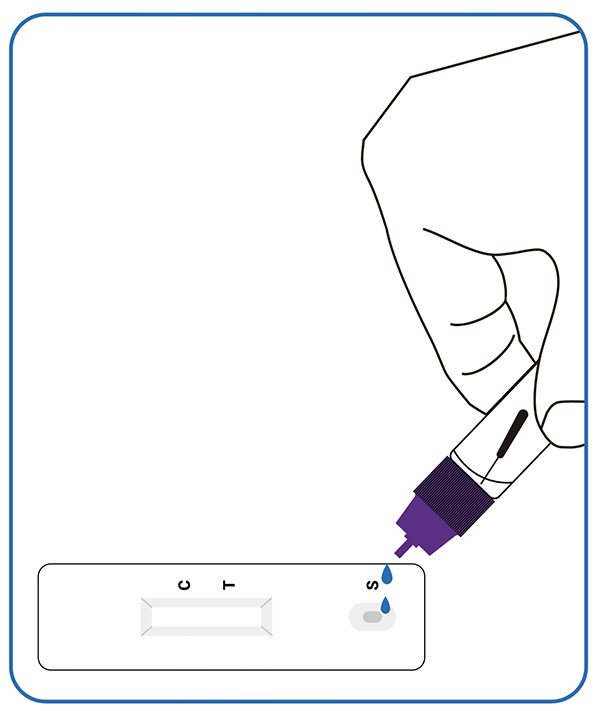
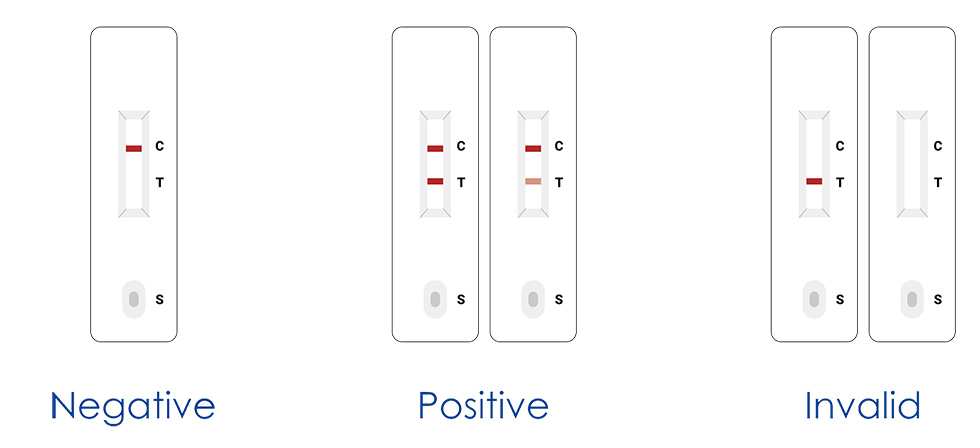
3. Lege exitum (15-20 min.)