Mycobacterium Tuberculosis INH Resistentia
Product Name
HWTS-RT002A-Mycobacterium Tuberculosis Isoniazid Resistentia Detection Kit (Fluorescens PCR)
Testimonium
Suspendisse FDA
Epidemiologia
Isoniazid, clavis anti-tuberculosis pharmacum anno 1952 introductum, unum ex efficacissimis medicamentis ad tractationem activam tuberculosis coniunctam et unum pharmacum ad tuberculosis latentem.
KatG gene principale est descriptam catalasi-peroxidasem et katG gene mutationis synthesim acidi mycolici parietis cellae promovere potest, bacteria isoniazid renitentem facit.Expressio KatG negative connectitur cum mutationibus in INH-MIC, et diminutio 2-triplex in expressione katG resultat paulo ampliore 2-ovili augmento in MIC.Alia causa resistentiae isoniazidorum in mycobacterio tuberculosis occurrit, cum basis insertionis, deletionis vel mutationis occurrit in InhA gene loco mycobacteri tuberculosis.
Channel
| ROX | inhA (-15C>T) situs· |
| CY5 | katG (315G>C) site |
| VIC (HEX) | IS6110 |
Technical Parameters
| Repono | ≤-18℃ in tenebris |
| Fasciae vita | XII mensibus |
| Specimen Type | Sputum |
| CV | ≤5.0% |
| LoD | 1 103Bacteria/mL* |
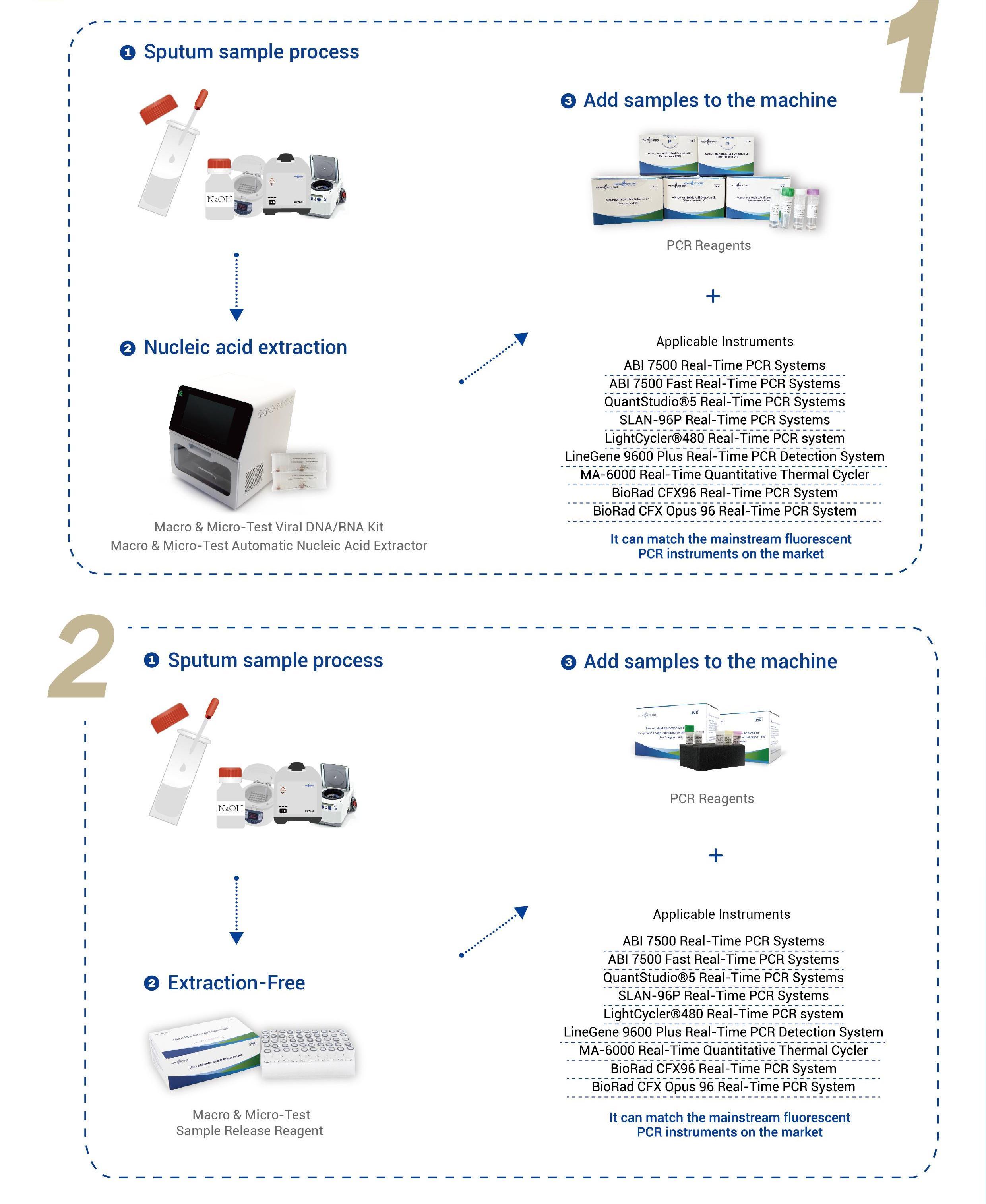
| Specification | Reactivitas in cruce nulla cum mutationibus situum resistentium quattuor medicamentorum (511, 516, 526 et 531) de rpoB gene extra detectionem range detectionis ornamentum. Instrumenta applicabilia: Acta Biosystems (VII)D Real-time PCR Systems Acta Biosystems (VII)D Fast Verus-Tempus PCR Systems QuantStudio®5 Real-Time PCR Systems SLAN-96P Real-time PCR Systems LightCycler®480 Real-time PCR systema LineGene 9600 Plus Real-time PCR Detection System MA-6000 Real-time Quantitatis Thermal Cycler BioRad CFX96 Real-time PCR System BioRad CFX Opus 96 Real-time PCR System |