SARS-CoV-2/influenza A /influenza B
Product nomen
HWTS-RT148-SARS-CoV-2/influenza A /influenza B Nucleic Acidum Detection Compositum Kit (Fluorescens PCR)
Channel
| Nomen Channel | PER-MIX 1 | PER-MIX 2 |
| FAM Channel | ORF1ab gene | IVA |
| VIC/HEX Channel | Internum imperium | Internum imperium |
| CY5 Channel | N gene | / |
| ROX Channel | E gene | IVB |
Technical Parameters
| Repono | -18℃ |
| Fasciae vita | XII mensibus |
| Specimen Type | nasopharyngeal swabs et oropharyngeal swabs |
| Target | SARS-CoV-2 tria scuta (Orf1ab, N et E genes )/influenza A /influenza B |
| Ct | ≤38 |
| CV | ≤10.0% |
| LoD | SARS-CoV-2:300 Copies/mL influenza A virus:500 Copies/mL influenza B virus:500 Copies/mL |
| Specification | a) Crux test eventus demonstravit ornamentum coronavirus hominum SARSr- CoV, MERSr-CoV, HcoV-OC43, HcoV-229E, HcoV-HKU1, HCoV-NL63, virus syncytialis respiratorii A et B, parainfluenza virus 1, 2 et 3, rhinovirusA, B, C, adenovirus 1, 2, 3, 4, 5, 7 et 55, metapneumovirus humanus, enterovirus A, B, C, D, virus cytoplasmicum pulmonis humanum, EB virus, boa virus cytomegalovirus Humani; rotavirus, norovirus, mumps virus, varicella zoster virus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium albicans tuberculosis, Candidasis, Mycobacterium tuberculosis, Candidas. crux reactionem inter Pneumocystis yersini et Cryptococcum neoformans. b) Impedimentum anti- quitatis: mucinum selectum (60mg/mL), sanguinem humanum 10% (V/V), diphenylephrinum (2mg/mL), hydroxymethylzolinum (2mg/mL), sodium chloridum (conservativa continens) (20mg/mL); beclomethasone (20mg/mL), dexamethasone (20mg/mL), flunisone (20μg/mL), triamcinolone acetonido (2mg/mL), budesonide (2mg/mL), mometasone (2mg/mL), fluticasone (2mg/mL), histaminum hydrochloridum (5mg/mL), α-Interferonium (800IU/mL), zanamivir (20mg/mL), ribavirin (10mg/mL), oseltamivir (60ng/mL), pramivir (1mg/mL), lopinavir (500mg/mL), ), ritonavir (60mg/mL), mupirocin (20mg/mL), azithromycin (1mg/mL), ceprotene (40μg/mL), Meropenem (200mg/mL), levofloxacin (10μg/mL) et tobramycin (0.6mg/mL) .Eventus ostendit substantias impedimentis ad has concentrationes nullas impedimentum esse responsionem deprehensionis eventuum pathogensum. |
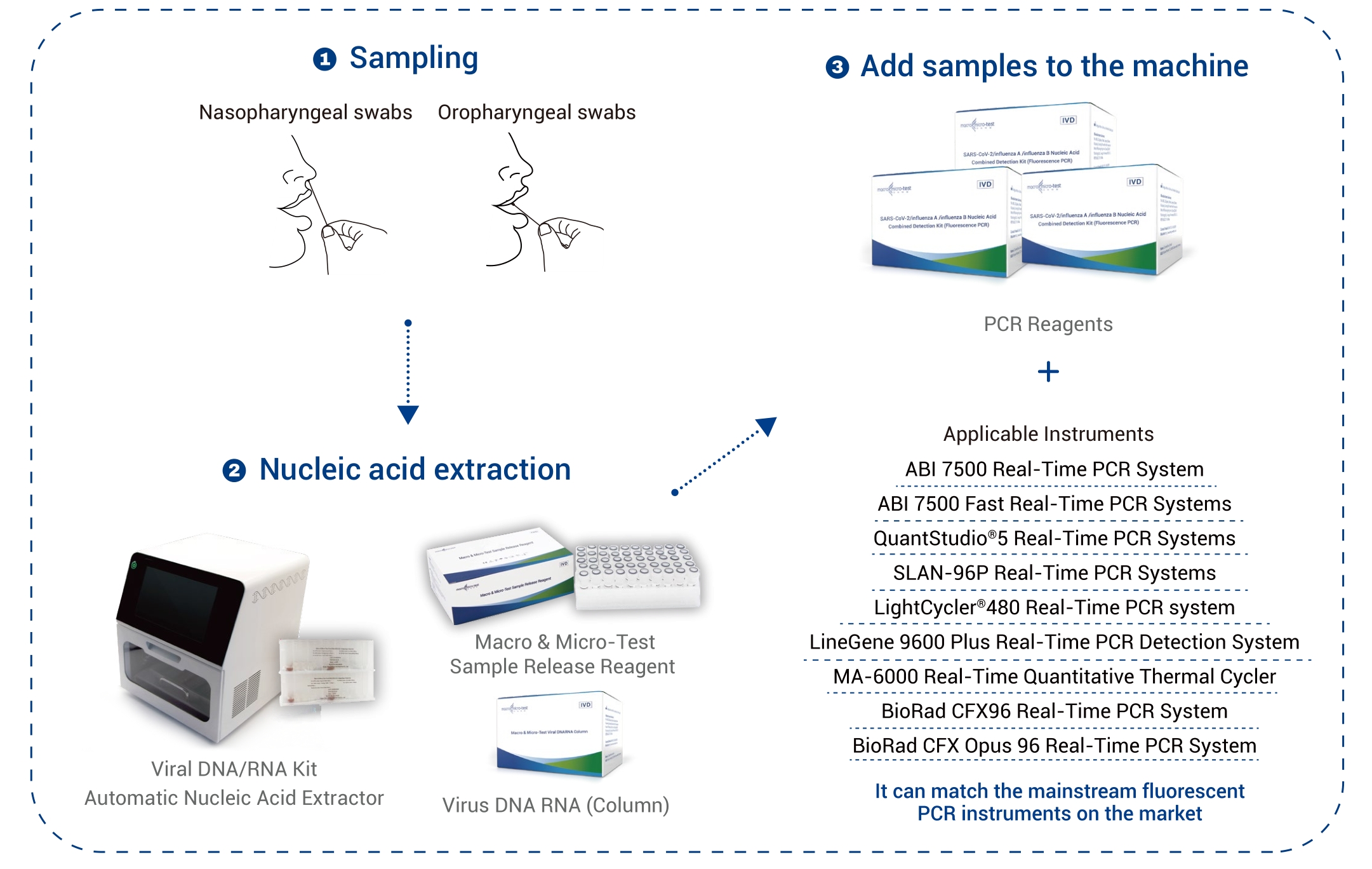
| Applicabilis Instrumenta | Acta Biosystems (VII)D Real-time PCR Systems Acta Biosystems (VII)D Fast Verus-Tempus PCR Systems SLAN ®-96P Real-time PCR Systems QuantStudio™ 5 Real-Time PCR Systems LightCycler®480 Real-time PCR system LineGene 9600 Plus Real-time PCR Detection System MA-6000 Real-time Quantitatis Thermal Cycler BioRad CFX96 Real-time PCR System BioRad CFX Opus 96 Real-time PCR System |
Summa PCR Solutio