SARS-CoV-2/influenza A/influenza B
Nomen producti
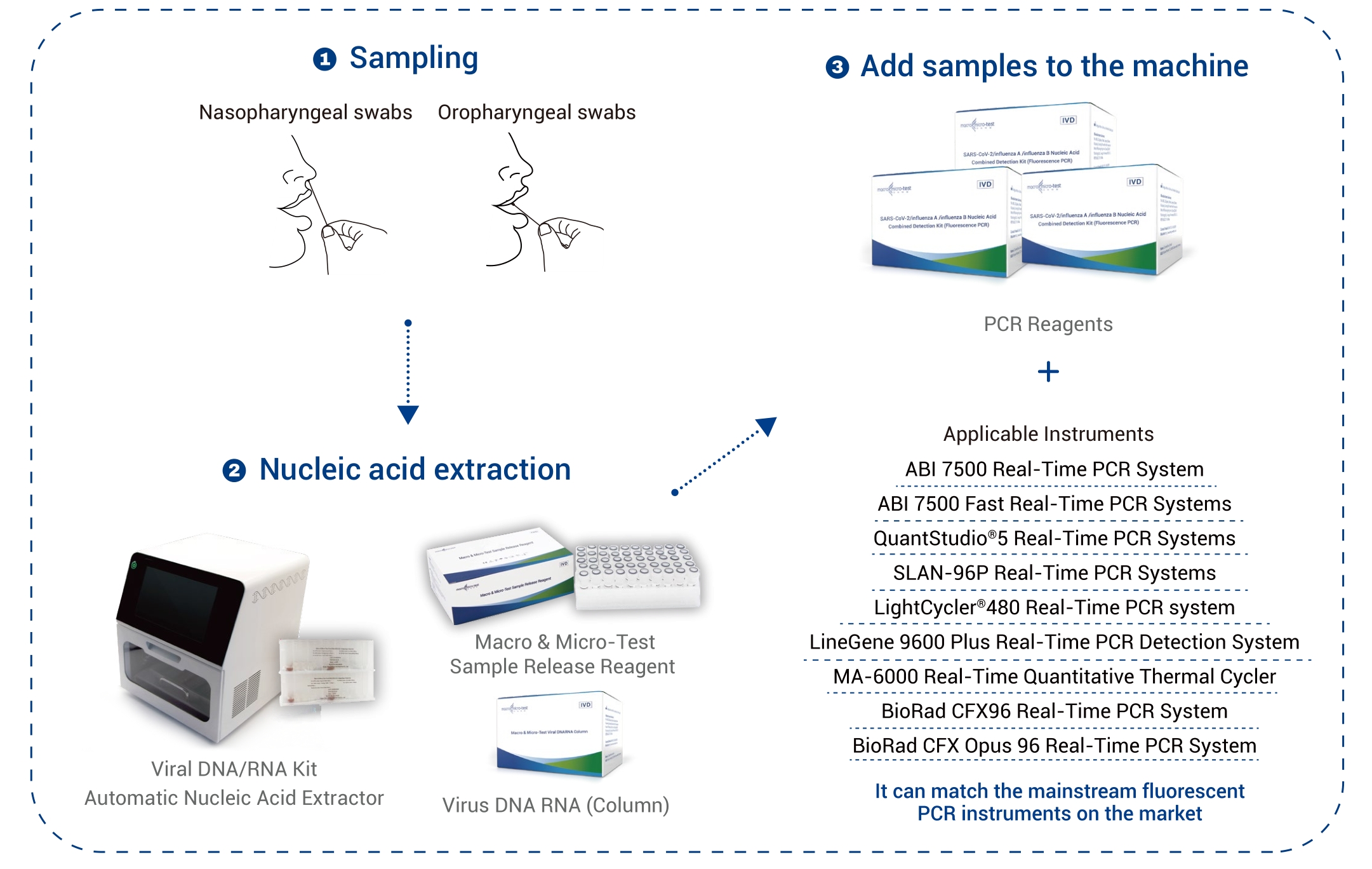
HWTS-RT148-SARS-CoV-2/influenza A/influenza B Instrumentum Detectionis Acidi Nucleici Combinatum (PCR Fluorescens)
Canalis
| Nomen Canalis | PCR-Mix 1 | PCR-Mix 2 |
| Canalis FAM | Genum ORF1ab | IVA |
| Canalis VIC/HEX | Imperium internum | Imperium internum |
| Canalis CY5 | Genum N | / |
| Canalis ROX | Gen E | IVB |
Parametri Technici
| Depositorium | -18℃ |
| Tempus conservationis | Duodecim menses |
| Typus Speciminis | Peniculi nasopharyngei et peniculi oropharyngei |
| Scopus | Tres scopi SARS-CoV-2 (Orf1ab, gena N et E) / influenza A / influenza B |
| Ct | ≤38 |
| CV | ≤10.0% |
| Limitatio Detectionis (vel Limitatio Detectionis) | SARS-CoV-2:300 exemplaria/mL Virus influenzae A: 500 exemplaria/mL Virus influenzae B: 500 exemplaria/mL |
| Specificitas | a) Resultata probationum transversalium demonstraverunt apparatum compatibilem esse cum coronavirus humano SARSr-CoV, MERSr-CoV, HcoV-OC43, HcoV-229E, HcoV-HKU1, HCoV-NL63, virus syncytiale respiratorium A et B, virus parainfluenzae 1, 2 et 3, rhinovirus A, B et C, adenovirus 1, 2, 3, 4, 5, 7 et 55, metapneumoviro humano, enterovirus A, B, C et D, virus pulmonare cytoplasmaticum humanum, virus EB, virus morbillorum. Cytomegalovirus humanum, rotavirus, norovirus, virus parotitidis, virus varicellae zoster, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus. fumigatus, Candida albicans, Candida glabrata. Nulla reactio cruciata inter Pneumocystis yersini et Cryptococcus neoformans observata est. b) Facultas contra interferentiam: mucinum selectum (60mg/mL), 10% (V/V) sanguinis humani, diphenylephrinum (2mg/mL), hydroxymethylzolinum (2mg/mL), natrii chloridum (conservativum continens) (20mg/mL), beclomethasonum (20mg/mL), dexamethasonum (20mg/mL), flunisonum (20μg/mL), triamcinolonum acetonidum (2mg/mL), budesonidum (2mg/mL), mometasonum (2mg/mL), fluticasonum (2mg/mL), histamini hydrochloridum (5mg/mL), α-Interferonum (800IU/mL), zanamivirum (20mg/mL), ribavirinum (10mg/mL), oseltamivirum (60ng/mL), pramivirum (1mg/mL), lopinavirum (500mg/mL), ritonavirum. (60mg/mL), mupirocinum (20mg/mL), azithromycinum (1mg/mL), ceproteinum (40μg/mL), Meropenemum (200mg/mL), levofloxacinum (10μg/mL) et tobramycinum (0.6mg/mL). Resultata demonstraverunt substantias interferentes in supradictis concentrationibus nullam responsionem interferentem ad resultata detectionis pathogenorum habuisse. |
| Instrumenta Applicabilia | Systema PCR Temporis Realis 7500 Biosystems Applied Systema PCR Temporis Realis Celeris Applied Biosystems 7500 SLAN ®-96P Systema PCR Temporis Realis Systema PCR Temporis Realis QuantStudio™ 5 Cyclus Lucis®Systema PCR in Tempore Reali 480 Systema Detectionis PCR in Tempore Reali LineGene 9600 Plus MA-6000 Cyclator Thermalis Quantitativus Temporis Realis Systema PCR Temporis Realis BioRad CFX96 Systema PCR Temporis Realis BioRad CFX Opus 96 |
Solutio PCR Totalis